Clinical Trials at Drexel Medicine


Why Participate in a Clinical Trial at Drexel?
Drexel University College of Medicine's clinical research program seeks to improve patient care through innovative approaches and new findings. By evaluating medicines, therapies and diagnostic tools, clinical research drives discoveries in health promotion and disease prevention to improve health.
Clinical Trials Open to Enrollment
The College of Nursing and Health Professions* is enrolling participants in a study to learn if brain activity during eating can predict weight loss.
The study consists of a total of five visits at Drexel University involving consumption of food and assessment of brain activity. Each visit will be on separate days plus FREE online weight loss intervention for six months.
- Are you between 18-65 years of age?
- Do you have no allergies to dairy, wheat, soy, nuts, tomatoes or corn?
- Do you wish to be part of a free weight loss study with brain activity measurement?
For participating in the study you will receive:
- $150 compensation ($30 for each session)
If you think you may be eligible or for more information contact the Nasser Eating Behavior Laboratory at 267.359.6287 or FoodBrainStudies@drexel.edu.
*This research is conducted by a researcher who is member of Drexel University.
Download flyer
Back to Top
Who can participate?
- Individuals with mild dementia and their care partners
What is involved?
- Care Partner Support & Training: Engage in a 4-week online mindful movement and support groups focused on nonverbal communication and self-care.
- Mindful Movement Program for Care Pairs: Weekly 1-hour online sessions blending mindfulness, multisensory exploration and shared movement for 8 weeks. Enhance connection, well-being and relationship quality.
- Complete surveys and participate in an interview to share your experiences.
Reach out for more information or to join our study.
Back to Top
We want to hear from you about being treated differently because of who you are and how it affects your health.
Description: If you are interested in participated, please visit https://drexel.qualtrics.com/jfe/form/SV_3QPy1osLBz74GF0?Source=directorg (online survey and compensation/encuesta en linea y compensacion)
Contact: treatyou@drexel.edu
Download Flyer / Descargar Folleto
Back to Top
PI: Wei Du, MD
Sponsor: Medical Marijuana Research Center, Drexel University
Description: We are enrolling participants in a study to learn how medical marijuana affects the health of people living with human immunodeficiency virus (HIV). 1) We are particularly interested in individuals with HIV-related nerve pain or 2) post-traumatic stress disorder (PTSD), but are also recruiting individuals without nerve pain or PTSD for comparison purposes.
Eligibility Criteria:
- You have HIV
- You are 18 years or older and have a valid PA Medical Marijuana Card
- You plan to begin taking medical marijuana for your medical conditions (HIV, PTSD, HIV-related nerve pain) but have not yet started
- You can afford medical marijuana and are willing to use Verano products
Contact: MedicalCannabisStudy@drexel.edu or call 215.762.7770.
Back to Top
PI: Amy Althoff, MD
Sponsor: NA
Description: This is a non-research expanded access Investigational New Drug protocol that allows for the use of Tecovirimat for monkeypox infections. The link to the protocol is: https://www.cdc.gov/mpox/hcp/clinical-care/tecovirimat.html
Eligibility Criteria: Be a PCCP patient over the age of 18 showing signs of monkeypox infection
Contact: Carolyn Edwards (267.507.6768)
Clinical and Translational Research Support Core of the Comprehensive NeuroHIV Center
PI: Brian Wigdahl, PhD
Sponsor: NIH
Description: We invite you (English or Spanish speaking) to take part in a research study because you are an HIV-1 infected patient over the age of 18, or you are an uninfected patient who will be a part of our control group.
Lo invitamos a participar en un estudio de investigación porque es un paciente infectado por el VIH-1 y es mayor de 18 años o es un paciente no infectado que formará parte de nuestro grupo de control.
Eligibility Criteria: Subjects 18 years of age and older that are HIV positive
Contact: Kim Malone - 267.507.6645 or Shinika Tillman - 267.507.6609
Back to Top
Learn More
Clinical trials are research studies involving human participants that evaluate a new medical approach, device, drug or other treatment. They are performed with people who have volunteered to help answer a specific health question. For example, a clinical trial might determine whether a certain drug or treatment prevents a certain disease or condition.
Clinical trials are conducted in four phases, with each phase involving a larger number of participants.
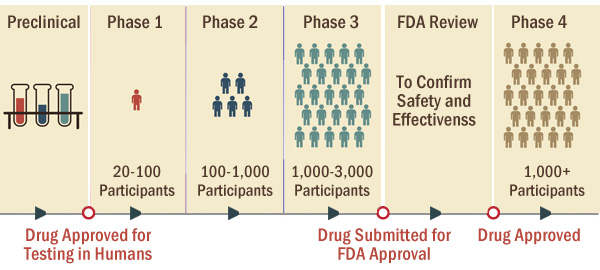
Back to Top
Each clinical trial contains enrollment guidelines about who can and cannot participate. These guidelines are maintained to ensure the safety of participants and potential participants. They are also used to ensure that the purpose of the study is effectively answered.
The guidelines are never used to personally reject a person from a clinical trial. Each clinical trial will clearly state both inclusion and exclusion criteria. Inclusion criteria are a list of the factors that allow someone to participate. Exclusion criteria are a list of the factors that will disqualify a person from participation. In order to participate, a person must have all the factors listed in the inclusion criteria and not have any of the factors in the exclusion criteria. Both sets of criteria may contain factors based on age, gender, ethnicity, disease or condition (or lack of), fertility and other health factors.
Back to Top
This will depend on the particular trial you seek to join. There are usually two types of costs in a clinical trial: costs for treatment that are considered "standard of care" and research costs that are associated with the research activity.
"Standard of care" costs are those that would have been part of your medical treatment or care if you did not enroll in the clinical trial. These treatment costs include but are not limited to drugs, routine laboratory tests, x-rays, scans, surgeries, routine medical care, and physician charges. You or your insurance company will be responsible for these "standard of care" costs.
Research costs are usually paid for by the trial at no cost to you. However, you will still be responsible for any co-payments and/or deductibles. There also may be additional costs, such as personal time and travel expenses, which may or may not be covered by a clinical trial. Please be aware that each trial is unique. The study team will go over the costs of participation with you before you agree to participate.
Back to Top
One way to gain access to promising new interventions is to participate in a clinical trial.
Although participating may not always be beneficial, there is reason to believe participating will be as good as or better than the current standard of care. The current standard of care was the result of past clinical trials, and a new research intervention may become the standard of care in the future.
All those receiving care are benefiting from someone who participated in a clinical trial in the past. By participating in a clinical trial today, you are helping advance medicine and, therefore, helping other patients in the future.
However, all clinical trials have risks. The new intervention being tested may have unknown side effects or other risks, which may or may not be worse than those from existing standard of care interventions. The research intervention may not work for you, even if it helps others. There may be inconveniences such as more frequent office visits and testing, as well as time and travel commitments.
The benefits of a well-designed clinical trial are:
- The participant may gain access to new research treatment that may not be widely available;
- The participant may obtain expert medical care at leading medical centers; and
- The participant contributes to medical research and future medical treatments.
The risks of participating in a clinical trial are:
- The patient may experience unpleasant, serious and even life-threatening side effects;
- The treatment may not be effective; and
- Participation may require more time and attention than receiving standard care (e.g., travel to the research site, additional tests or treatments, hospital stays, complex dosage).
Back to Top
Consent
Before agreeing to participate, you will be asked to review and sign an informed consent document with your doctor. The consent form will explain the study's purpose, procedures and possible risks and benefits of participation in detail. If new benefits, risks or side effects are found during the course of the trial, you will be told about them so that you can decide whether or not you want to continue participating. Taking part in a clinical trial is completely voluntary and you can choose to stop participating at any time for any reason. Your health care providers will continue to care for you according to current best practices and highest standards of care, regardless of whether or not you continue participation in a clinical trial.
Oversight
Local, federal and international laws protect clinical trial participants' physical safety and privacy. Several oversight committees approve and routinely monitor trials to make sure that the rights and safety of participants are protected. These committees have the ability to shut down a trial if it is deemed unsafe.
Back to Top
Clinical trials are funded (or sponsored) by an array of institutions, individuals and organizations, such as physicians, medical institutions, medical academic centers, foundations, pharmaceutical companies and governmental agencies. The National Institutes of Health (NIH), the Department of Defense (DoD), and the Department of Veterans Affairs (VA) are examples of governmental agencies that sponsor clinical trials.
Clinical trials can be conducted in various places including hospitals, universities, medical offices, community clinics and research clinics.
Back to Top